Del Mar Photonics
ANALYSIS OF BIOLOGICAL MOLECULES ON SURFACES USING STIMULATED DESORPTION
PHOTOIONIZATION MASS SPECTROMETRY
The ions were detected by drifting through the field free flight tube and
reaching a pair of chevron configuration microchannel plates (MCPs) (Del Mar
Ventures, San Diego, CA, USA) which magnified the signals by factors of 106
~107.
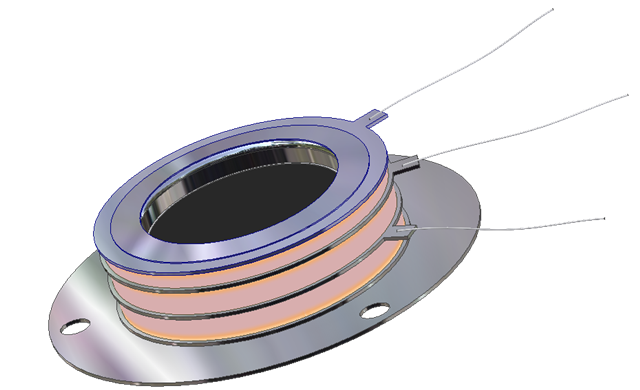 |
Open Microchannel Plate Detector
MCP-MA25/2 -
now in stock!
Microchannel Plate Detectors MCP-MA series are an open MCP detectors
with one or more microchannel plates and a single metal anode. They are intended
for time-resolved detection and make use of high-speed response properties of
the MCPs. MCP-MA detectors are designed for photons and particles detection in
vacuum chambers or in the space.
MCP-MA detectors are used in a variety of applications including UV, VUV and EUV
spectroscopy, atomic and molecular physics, TOF mass–spectrometry of clusters
and biomolecules, surface studies and space research.
MCP-MA detectors supplied as a totally assembled unit that can be easily mounted
on any support substrate or directly on a vacuum flange. They also can be
supplied premounted on a standard ConFlat flanges.
buy online -
ask for research discount!
|
Rapid growth of biological, environmental, clinical, pharmaceutical and material
sciences have led to dramatically increased demands for chemical and structural
information regarding organic/biological molecules from complex systems. Many
analytical tools have been developed and utilized to characterize and analyze
biomolecules.1-6 Mass spectrometry (MS) is one of the most successful and
popular techniques for the analysis of a broad range of analytes of interest. It
uses different ionization methods to convert analytes into ions and separate
them according to their mass-to-charge ratio (m/z) by various mass analyzers.
Combined with separation techniques such as gas chromatography (GC), high
performance liquid chromatography (HPLC) and capillary electrophoresis (CE),
mass spectrometry is a very powerful tool to detect many compounds from
relatively complicated systems. By employing proper ionization methods, mass
spectrometry enables qualitative and quantitative analysis of many molecules
with high sensitivity. By coupling high resolution mass analyzer with specific
ionization sources, mass spectrometry provides accurate molecular mass and
fragmentation data which allows the determination of elemental composition and
chemical structure of analytes. Therefore, mass spectrometry is playing an
increasing pivotal role in biological related research and applications. The
further improvements of mass spectrometry in sample preparation,
instrumentation, desorption, and ionization methods will be highly beneficial to
high-throughput analysis of biological molecules in complex systems.
Stimulated desorption photoionization mass spectrometry (SD/PI MS) has been
widely used in bimolecular analysis in recent years. This technique desorbs
analyte molecules by photons or electrons and ionizes them by direct or indirect
energy transfer. According to the different desorbing and ionizing sources,
stimulated desorption photoionization mass spectrometry could be divided into
three main categories: i) laser desorption/ionization mass spectrometry (LDI MS)
in which both desorption and ionization are promoted by one laser; ii) laser
desorption photoionization mass spectrometry (LD/PI MS) in which a laser desorbs
the analytes and another laser ionizes the desorbed molecules by single photon
ionization (SPI) or multiphoton ionization (MPI); iii) electron stimulated
desorption photoionization mass spectrometry (ESD/PI MS) in which an electron
beam is used to desorb molecules from the target surface and a laser beam is
used to ionize the desorbed species.
The introduction of matrix-assisted laser desorption/ionization mass
spectrometry (MALDI-MS) in the 1980s 7, 8 has dramatically improved the ability
of stimulated desorption photon ionization mass spectrometry. In MALDI MS,
samples are usually prepared by mixing with an excess amount (~104 fold) of
matrix molecules that absorb laser energies. The use of matrix molecules is
critical for success of the laser desorption/ionization. It serves to isolate
analyte molecules from each other, to absorb the intense laser radiation, to
vaporize and propel the analyte molecules into the gas phase, and subsequently
to ionize the neutral analytes in the plume of the excited-state matrix
immediately above the sample target.9 By using a time-of-flight mass
spectrometer with a nearly unlimited mass range, MALDI MS has successfully
detected large biomolecules and synthetic polymers up to 1.5 million Daltons
(Da).10 In addition, MALDI MS also provides soft ionization (i.e. the parent
ions dominate the mass spectrum with little or no fragmentation), high detection
sensitivity and fast sample analysis. MALDI MS has become one of the
cornerstones of the revolution in bioanalysis.8, 11-13
Although MALDI MS has been remarkably successful in making many advances in
various fields, some the limitation still hinders its full development and
application. A complete understanding of the MALDI phenomenon mechanism does not
yet exist,14 which greatly affects the optimization of MALDI. The effectiveness
of the matrix material is also not always intuitively apparent. Thus, the matrix
selection is often obtained after an exhaustive empirical search.15 Poor
shot-to-shot and sample-to sample reproducibility resulting from the crystalline
matrix is another issue that must be dealt with for the improvement of MALDI
performance. Finally and most importantly, MALDI produces a large amount of
matrix background ions below m/z 600, which makes it impossible to analyze small
molecules.
Increasing demands for high throughput methods in drug discovery, and
biotechnology as well as analysis of complex mixtures in high salt matrices and
buffers, has created great interest in utilizing the full power of LDI over the
entire mass range of interest.16
In the studies presented in this dissertation, a versatile ultrahigh vacuum
(UHV) analytical system was designed and constructed for the analysis of small
organic/biological molecules using different forms of stimulated desorption
photoionization mass spectrometry (details are described in chapter 2). The
approach pursued in this thesis work is to carry out detailed studies which
concentrate on the 3
surface chemistry and physics governing non-thermal desorption. The combination
of this approach with sensitive laser detection schemes developed by the atomic
and molecular physics communities has provided the paths for advancing
bioanalytical techniques. Specifically, surface-assisted desorption/ionization
(SALDI MS), laser desorption single photon ionization mass spectrometry (LD/SPI
MS) and electron stimulated desorption single photon ionization mass
spectrometry (ESD/SPI MS) methods were developed to characterize small thermally
labile molecules in different sample environments. The high sensitivities of
SALDI MS, LD/SPI MS, as well as ESD/SPI MS and the generalities of their
applications suggests that these techniques may be used as widespread tools for
the detection of small analytes in complicated biological samples.
SALDI-MS has been newly developed as a supplemental technique for MALDI MS. It
utilizes specific target substrates with porous structures and high photon
absorptivity to retain samples and generate soft ionization. Because this method
does not add matrix molecules, it produces a clean mass spectrum in the low-mass
range for easy characterization of small molecules. In chapter 3, SALDI MS on
three different surfaces were studied to provide useful guides to its
applications. The effectiveness of SALDI MS in analysis of the amino acids,
small peptides, and organoselenium metabolites were also investigated to explore
its potential of analyzing various molecules in complex systems. The amino
acids, dipeptides and organoselenium are typical small molecules with great
significance in many aspects and the fast and sensitive analysis of these
molecules has been very challenging. In addition, the analysis of these
molecules usually involves great amounts of sample which require a
high-throughput method. Therefore, traditional techniques such as HPLC-MS and
GC-MS which suffer from time consuming operation and methods development that
often can not provide satisfactory analysis. The good performance of SALDI MS on
analyzing amino acids, dipeptides and organoselenium metabolites illustrate a
potential approach for simple, fast and sensitive analysis of small molecules.
Although SALDI MS has been successfully applied in many applications, its
mechanism is not clear. To fully understand the mechanisms of SALDI MS, the
effects of surface morphology, sample solvent, surface storage, surface
temperature, sample acidity have been investigated and discussed in chapter 4. A
proposed mechanism is given and this could lead the optimization of SALDI MS.
Based on the fact that natural desorption yields are typically 1000~10,000 fold
greater than the ion yields and two-step laser desorption photoionization
technique could provide better control in both the desorption and ionization
processes, the LD/SPI MS method was developed for sensitive analysis of
metabolites in human urine. This approach uses an ultraviolet (UV) laser to
desorb intact neutral molecules and a vacuum ultraviolet VUV laser to ionize the
desorbed neutral molecules by single photon ionization. The details of LD/SPI MS
are discussed in chapter 5. This technique provides more efficient detection
than secondary ion mass spectrometry (SIMS), direct non-resonant laser
ionization (LDI), MALDI and SALDI. An HPLC-MS/MS method was also created for the
analysis of organoselenium metabolites and it provided results similar to those
obtained using LD/SPI MS. This study demonstrates the viability of matrix free
LD/SPI MS for molecular characterization and quantitive analysis of biological
metabolites in the m/z 10 ~ 600 range that are present in complex biological
fluids.
By taking advantage of high efficiency, high sensitivity and uniform selectivity
of single photon ionization, deoxyribonucleic acid (DNA) damage induced by
low-energy electrons was investigated by ESD/SPI MS. This is discussed in detail
in chapter 6. DNA damage caused by irradiation is of great importance to the
application of radiobiology, public health, and clinical treatments. To
understand the genotoxic effects and cell damage due to secondary species of
high-energy radiation, the role of transient negative ions (TNI) and the
specificity in LEE-DNA damage were studied by examining the neutral product
yields using ESD/SPI MS. The neutral yields as a function of incident electron
energy were also correlated with the SSBs and DSBs measured using
post-irradiation gel electrophoresis. The results indicate that resonances
involving the oxygen of the phosphate-sugar linkages and the surrounding water
molecules may contribute to the DNA damage. Careful measurements of the role of
intrinsic water and any sequence dependences of the strand break probability are
still underway.
Another feature of this home-build TOF was its ability of obtaining mass
spectrometric imaging of analyte ions by using a MCP/phosphor screen (MCP-IFP46/2;
Del Mar Ventures, San Diego, CA, USA) as an imaging detector. The TOF was
mounted on a special six-inch flange with a view port. A supporting plate was
designed to hold the MCP/phosphor screen detector right above the window. A
high-speed cooling CCD camera (Sensicam QE; Cook Corp., Auburn Hills, MI, USA)
was focused on the P46 phosphor screen which emitted fluorescence (490 ~ 620 nm)
with a fast 90%-to-10% decay (300 ns). By using proper pulse sequence and image
collection parameters, the images of different ions could be recorded by the CCD
camera. At the same time, the voltage change on the second MCP plate which
reflected the flight time and intensity of detected ions could also be obtained
by utilizing an oscilloscope through a RC circuit (Figure 2-3). Therefore, both
of the whole mass spectrum and images of individual ions were able to be
observed by this imaging TOF mass spectrometer (Figure 2-4).
full article
Microchannel Plates, Detectors
and Imaging Systems
Examples of research applications:
Studies of the atomic clusters at the
University of Virginia -
Amber Post
Featured MCP customer:
The Castleman Group at PSU
MCP home -
MCP references
MCP-GPS-46/2-CF6" Open
MCP imaging detector mounted on CF6" flange -
MCP-GPS and
MCP-IFP imaging detectors
MCP-MA -
Detecting short proton
beam from a picosecond CO2 laser ionized H2 plasma
MCP-MA25/2 are used in aSPECT to
study the background
MCP setup for velocity map imaging apparatus
Microchannel Plate Detector (MCP) setup for Plasma Desorption Mass
Spectrometry (PDMS)
MCP detector for high resolution ion time-of-flight analysis for measuring
molecular velocity distributions
X-ray detection system based on the MCP/phosphor screen assembly
MCP + phosphorous screen for imaging of XUV radiation (14eV- 160-eV) in high harmonics experiments
Exchanging MCPs in Time-of-Flight detectors
Microchannel Plates, Detectors
and Imaging Systems - Open MCP-MA
- MCP-MA applications -
MCP-MA assembled -
Applications
 |
We are looking forward to hear from you and help you with
your optical and crystal components requirements. Need time to think about
it?
Drop us a line and we'll send you beautiful Del Mar Photonics mug (or
two) so you can have a tea party with your colleagues and discuss your
potential needs. |

Del Mar Photonics, Inc.
4119 Twilight Ridge
San Diego, CA 92130
tel: (858) 876-3133
fax: (858) 630-2376
Skype: delmarphotonics
sales@dmphotonics.com


